The Single Cell: Singularly Inspiring
At its simplest, the single cell might be described as just a water-filled oil bubble. But look closer and it gets awesomely complex. Inside this watery world, thousands upon thousands of unique chemical processes—from “burning” sugars to building new proteins—occur simultaneously, each without interfering with another.
Tiny teachers, large lessons
Human-made technologies, processes, and organizations—impressive as they are—have a long way to go to rival the performance and sustainability of the humble single cell. Many of the wish-list features we want in everything—from our increasingly compact devices to our increasingly sustainable companies—are common features of cells.
For example, cells are:
decentralized
energy efficient,
carbon neutral or negative,
water-based,
compact/lightweight,
multifunctional,
rechargeable,
recyclable/compostable,
organic,
composed of life-friendly materials, and
manufactured from the bottom up.
In addition, cells:
run on sunlight, minerals, and sugar,
store a lot of information in a small space,
operate at ambient temperatures and pressures, and
can themselves be used as building blocks for bottom-up assembly of complex systems.
Science is at a point now where we can peer ever deeper into cellular machinery with increasingly high resolution. That means we continue to gain a richer, more detailed understanding of nature’s cellular mechanisms and architecture and the deep principles and patterns underlying them. Armed with this information, we are poised, if we choose, to mimic these deep principles and patterns in developing next-generation human technologies that are both high-performing and sustainable.
Come discover what I find fascinating and inspiring about the single cell.
The outside
Let’s start with an urban analogy to describe the cell’s outer membrane. Rather than a simple, uniform enclosure like that of a water balloon, the outer cell membrane is actually as busy and diverse a landscape as midtown Manhattan. The cell membrane is chock-full of chemical structures. Some rise high above the outside of the cell like proteinaceous Empire State Buildings, while others sink below the membrane into the cell interior like underground parking garages, while still others conjure up manholes that span the membrane to allow passage of items between the surface and the interior. What do all these structures have in common? They are anchored fully or partially in the cell’s outer membrane itself. From there, it’s like a Disney animation comes to life where these structures change position, join the cell membrane, or leave it.
Unlike actual skyscrapers, garages, and manholes that anchor into solid ground or concrete foundations, cellular structures are constantly on the move because they are anchored into, and float along with, the component parts of the cell membrane itself. In other words, the cell membrane is more like a crush of commuters swarming through Grand Central Station at rush hour than a single piece of material.
Cell membrane and its components. Some components attach to the outer surface, others attach to the inner surface, while others permeate the membrane to form pores or channels. Together, they facilitate a chemical conversation within and between cells through the selective docking and undocking of molecules governed by their unique 3D shape. (Source: LadyofHats, Public domain, via Wikimedia Commons)
Consider first that each tiny component of the cell membrane has two parts. One part is electrostatic, like clothes clinging to each other in the dryer, while the other part is oily. The electrostatic parts of these components attract each other as do the oily parts (like drops of oil on water that ultimately find each other and aggregate into a single glob). But these are weak connections similar to the ease with which you can pull clothes apart joined by “static cling” or separate globs of oil in a bottle of salad dressing by shaking it. These are weak connections that easily form, separate, and re-form. This allows the cell membrane to be a dynamic, ever-changing surface where structural components can join or leave the cell membrane, or just change position.
The Inside
We’ve all driven past factories of one sort or another where the workhorses of modern society churn out ingredients for our everyday use, whether they be flavors in our foods, fragrances in our lotions, medicines in our pills, cleaners in our cupboards, fibers in our clothing, or the energy to power all of those production processes. Often, they are noisy, acrid eyesores operating under dangerously high temperatures and pressures. Imagine, then, a factory with all of these production processes located together under one roof! Chances are, you wouldn’t want such a facility in your own backyard because of the noise, stench, and potential danger of poisonous leaks, spills, or explosions.
Yet right there, within and below your skin, every single cell making up your body and the bodies of your family, friends, pets, and plants is doing exactly that—powering the processes that churn out the wide range of ingredients that your body needs for its everyday use. In fact, science estimates there are thousands or millions of chemical processes occurring per second in a single living cell.
Internal organelles in a typical animal cell. The building and breakdown of some molecules happen within the confines of these membrane-bound “devices” while others happen out in the open water of the cell’s internal salty ocean. The organelles themselves are built modularly from these same molecules. She’s a clever one, that Mother Nature! (Source: LadyofHats, Public domain, via Wikimedia Commons)
Among the ingredients produced by cells are the acids and proteins that extract nutrients and energy from your food, the chemicals that move your muscles, clean your blood, grow your hair, heal your wounds, interpret taste and smell, and power your brain to read this article and make myriad decisions. These processes are happening simultaneously in a watery environment at the same temperatures and pressures that we find comfortable, pleasant, and safe.
Chemical hieroglyphics
How do our cells manage to do all of this? How are all these processes able to occur in harmony under mild, life-friendly conditions? A large part of the answer is cross-communication. The internal workings of each cell, as well as cross-communication between cells, happens through one common language: chemistry. You might think ancient Egyptian hieroglyphs or Sanskrit is the oldest language on planet Earth, but think again. The oldest language, by far, is chemistry—the sending and receiving of chemical signals.
Chemical communication is one of the main functions of those structures that decorate the cell membrane. They communicate with the outside world by “recognizing” and docking (like hand-in-glove) with specific chemicals floating in the external environment or attached to the surface of a nearby cell (which notably is how the Coronavirus is able to find and infect our cells). Or they can excrete chemical signals through the pores in the membrane to send out chemical words and phrases to other cells, such as “stay away, or else” or “here I am, let’s build a new bacterial community (i.e., a biofilm) on the surface of these tasty lungs,” or “go that way to a source of food.”
Similar conversations are happening inside the cell that orchestrates the symphony of simultaneous processes. Without these chemical orchestrations, the inside of a cell would be as chaotic as an elementary school playground at recess. For example, chemical signals trigger enzymes to produce nutrients like amino acids. Once enough of that particular nutrient is produced, its high concentration signals the enzymes to go dormant until the next time more of this nutrient is needed. Built-in self-regulation!
Electric eels have a fascinating type of chemical communication they use when threatened. They have special cells that generate on-demand electric current by controlling the movement of simple mineral ions (e.g., sodium, potassium, and chloride) from one side of the membrane to the other. (Source: Shutterstock)
Another example of chemical communication is when an electric eel feels threatened. It sends chemical signals to an army of specialized internal cells that simultaneously release a torrent of ions across each cell’s membrane that add up to a powerful electric punch.
Cells not only DO chemistry, they ARE chemistry
Nature’s pretty clever. The same molecules that aid chemical processes, like digestion, are also used to build more complex cellular machinery. That’s like saying a factory producing bar soap, also uses those bars as the building blocks to build the equipment, which then produces the packaging for the soap! In other words, nature builds chemicals for a particular purpose but also uses them to build larger structures, which, in turn, become parts of even more complex cellular machinery. Worthy of a Mary Poppins lyric, these are called supramolecular complexes.
If this multi-use, hierarchically structured application of cellular chemicals wasn’t cool enough, you may be surprised to learn that these molecules and structures are essentially held together with the chemical equivalent of paper clips—LOTS of them. Nature only uses covalent bonds (the super glue of the cellular world) when absolutely necessary. By relying mostly on weak connections, the structures are more adaptable to changing conditions and can be easily disassembled when they are ready to be recycled and reused. In other words, at the cellular level, nature builds by maximizing the use of easily reversible weak bonds—she uses strong bonds judiciously. That’s pretty much the opposite of what we do in the human-designed world, which is one reason man-made materials like plastics and industrial chemicals don’t decompose as readily as natural materials do.
Slow cooking
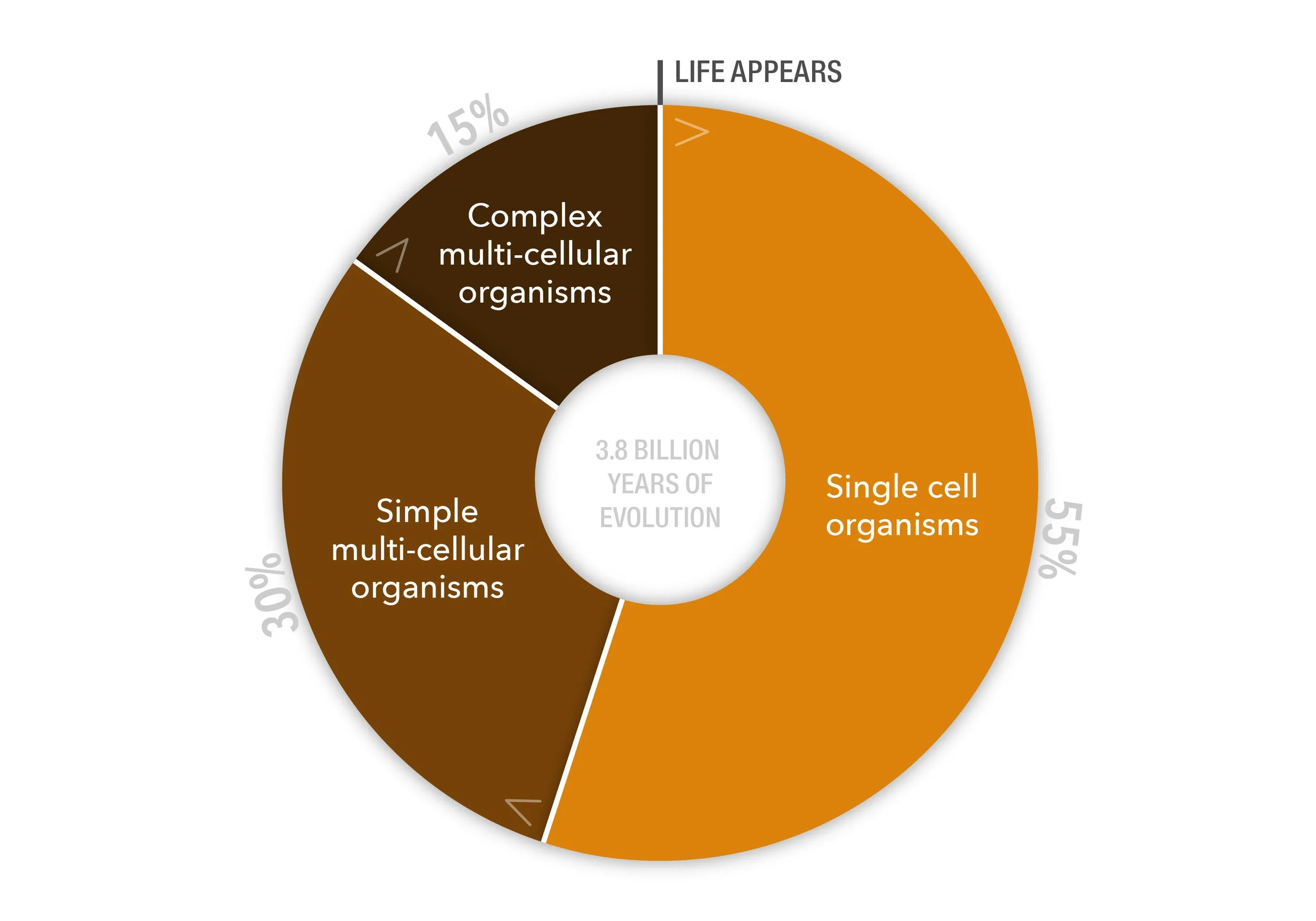
Nature didn’t come up with these elegant and sophisticated strategies overnight. It was a slow and iterative process. In fact, of the 3.8 billion years of life’s evolution on Earth, Mother Nature spent more than half (~55%) of that time perfecting the single cell. Only then did she move on to piecing them together to create simple, multi-cellular organisms and—only relatively recently—the explosion of the complex biological diversity we know today.
To perfect the single cell, Mother Nature spent the biggest chunk (just over half) of the 3.8 billion-year timeline of Life’s evolution on Earth. Only then did she give rise to the evolution of simple, multi-cellular organisms and—relatively recently—the explosion of the complex biological diversity we know today.
We humans—ourselves a product of nature’s design and engineering prowess—have at most spent 10,000 years developing technologies, but only a few hundred on advanced technologies like radios, x-ray imaging equipment, computers, portable electronic devices, plastics, a host of life-saving drugs, and an even bigger arsenal of life-destroying weaponry. Given the advances over the last 100 years, imagine where peace-time human technology might be after thousands or millions of years of R&D (assuming we don’t annihilate ourselves in the interim).
The good news is, we don’t have to wait that long if we stand on the shoulders of cellular giants by tapping into the knowledge that naturally surrounds us. Here are some examples of how scientists have done just that.
Coming full circle for a circular economy
Inspired by:
Coral polyp cells: Blue Planet invented a technology that mimics the way coral polyp cells take carbon dioxide and calcium dissolved in seawater to produce the hard, cement-like structures that make up coral reefs. The company captures carbon dioxide from smokestacks that would otherwise be released into the air and converts it to concrete.
Fungal cells: TAML Activators can break down toxic industrial chemicals that have lingered in the environment for nearly a century. These inventions mimic the working heart of enzymes that fungal cells use to break down even the toughest components of wood in dead trees.
Electric eel cells: While conventional batteries use minerals like lead and lithium to generate an electric current, electric eels have cells that use simple minerals like sodium, potassium, and chloride to accomplish the same task. Scientists have developed early mimics of these cells that could potentially power small devices.
Plant cells: Dye Sensitized Solar Cells (DSSC) mimic chloroplasts, the microscopic “devices” in plant cells, that use colorful pigments (the greens of summer and the reds and yellows of fall) to capture and convert the energy of sunlight into a flow of electrons (i.e., electricity). Plants use that flow to power the conversion of carbon dioxide inhaled through their leaves into sugars for growth. DSSCs use a colorful dye in concert with materials already used in commerce to generate electricity to power buildings and charge batteries.
What other performance and sustainability challenges do you think need to be addressed? Perhaps the inspiration for novel solutions lies in plain microscopic sight. B3.8 can help light the way.
I hope you’ve enjoyed this peek into the cellular world. If you want more, consider spending 3-5 days with me in nature digging into the deep patterns underlying the cell’s molecular harmonies. Sign up here to receive updates on the 2023 “Nature is Alive with Chemistry” workshop.